Chem 115 - General Chemistry I
Stoichiometry IX - Video Solution
Drierite is the brand name of a variety of desiccants made from anhydrous calcium sulfate. Each empirical unit of calcium sulfate can absorb two molecules of water. Drierite contains a trace of a color indicator that shows when it cannot absorb any more water. However, one can regenerate anhydrous calcium by heating it in a drying oven and driving off the water. What mass of water is released when 2.00 grams of CaSO4×2H2O are heated in a drying oven.
CaSO4×2H2O(s) 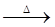 CaSO4(s) + 2 H2O(g)
CaSO4(s) + 2 H2O(g)
![]() CaSO4(s) + 2 H2O(g)
CaSO4(s) + 2 H2O(g)![]() CaSO4(s) + 2 H2O(g)
CaSO4(s) + 2 H2O(g)