P201
Menu
Class_Notes
December 8, 1998
Mentors: Send in Grades
(40%)
Sites without Mentors: Send in Work to:
Carl A. Rotter, Dept. of Physics Box 6315,
West Virginia U, Morgantown WV 26506-6315
Tests 1, 2, 3 -- if not taken, take by
12/15
Test 4 Opened in class tonight for a few
minutes of discussion
CD ROMs being sent to me to send to you via the Mentors.
For sites without mentors, notify me if you are teaching 9-12
and were registered for class.
Second Semester: January 12 - April 27
(no class on March 30 and April 6)
Title SPTP: CATS Conceptual Physics
CRN 15292
Course Phys 201B
Section 6W1
Hours 3.0
Day T 16:00-18:50
Bldg CERC... Morgantown
Satellite Telstar 4... Channel 20 89degrees west.
Conclusions to Work and Energy... some
Applications:
Work done by Human Heart
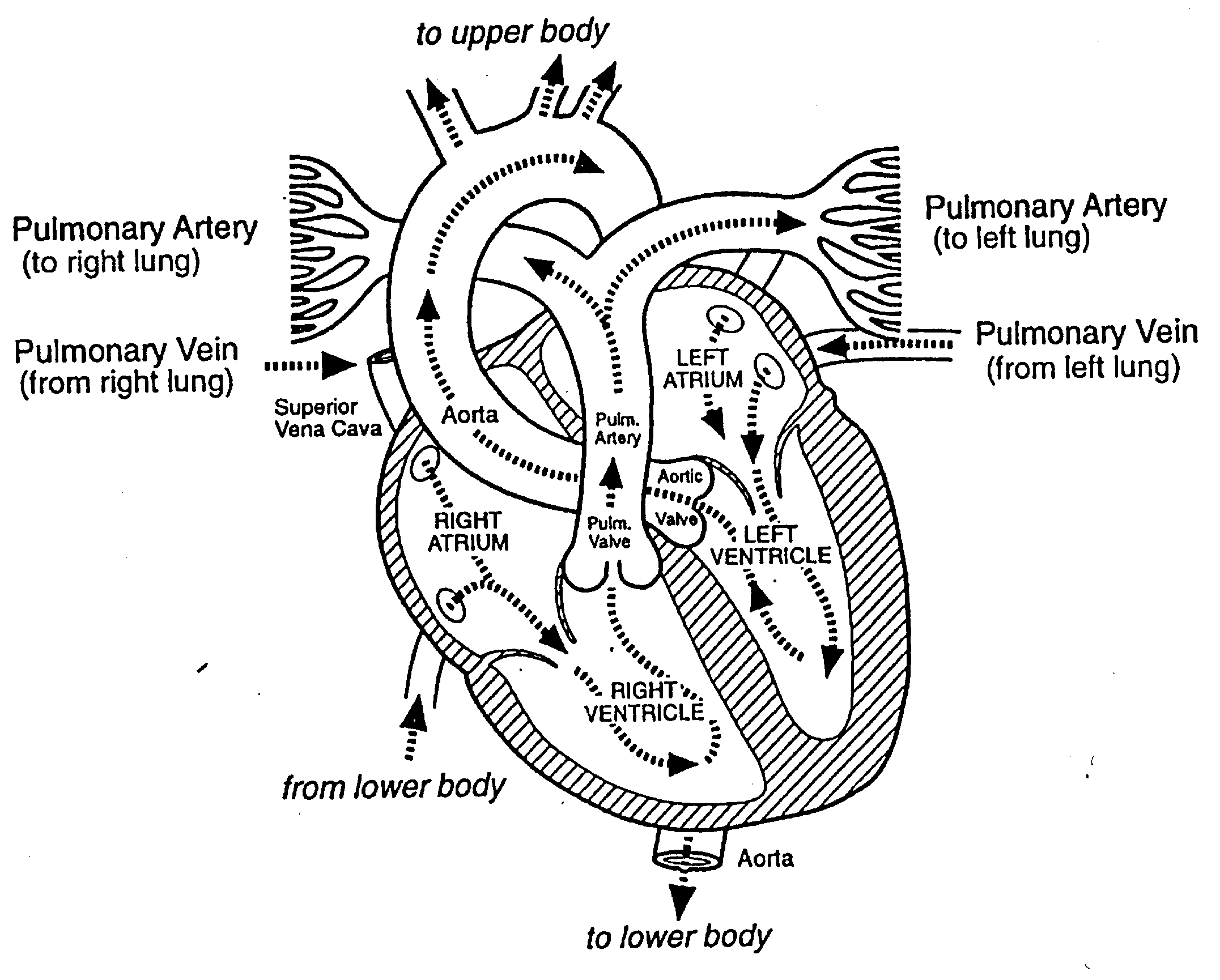
Work done by heart in each contraction
Assumptions: Pumping 5 Liters/minute,
gauge pressure in left ventricle is 100 mm Hg... gauge pressure
in right ventricle is 20 mm Hg... heart rate 1 contraction/second.
Left Ventricle:
WLV
= PLV DV LV = (1.33
x 104 N/m2)(83 x 10-6m3)
=
1.1J
Right Ventricle:
WRV
= PRV DVRV = (0.27
x 104 N/m2)(83 x 10-6m3)
=
0.2 J
Total work/contraction =
1. 3 Joule
Total power of heart = 1. 3 J/s = 1. 3 Watt
Work and Heat in an engine:
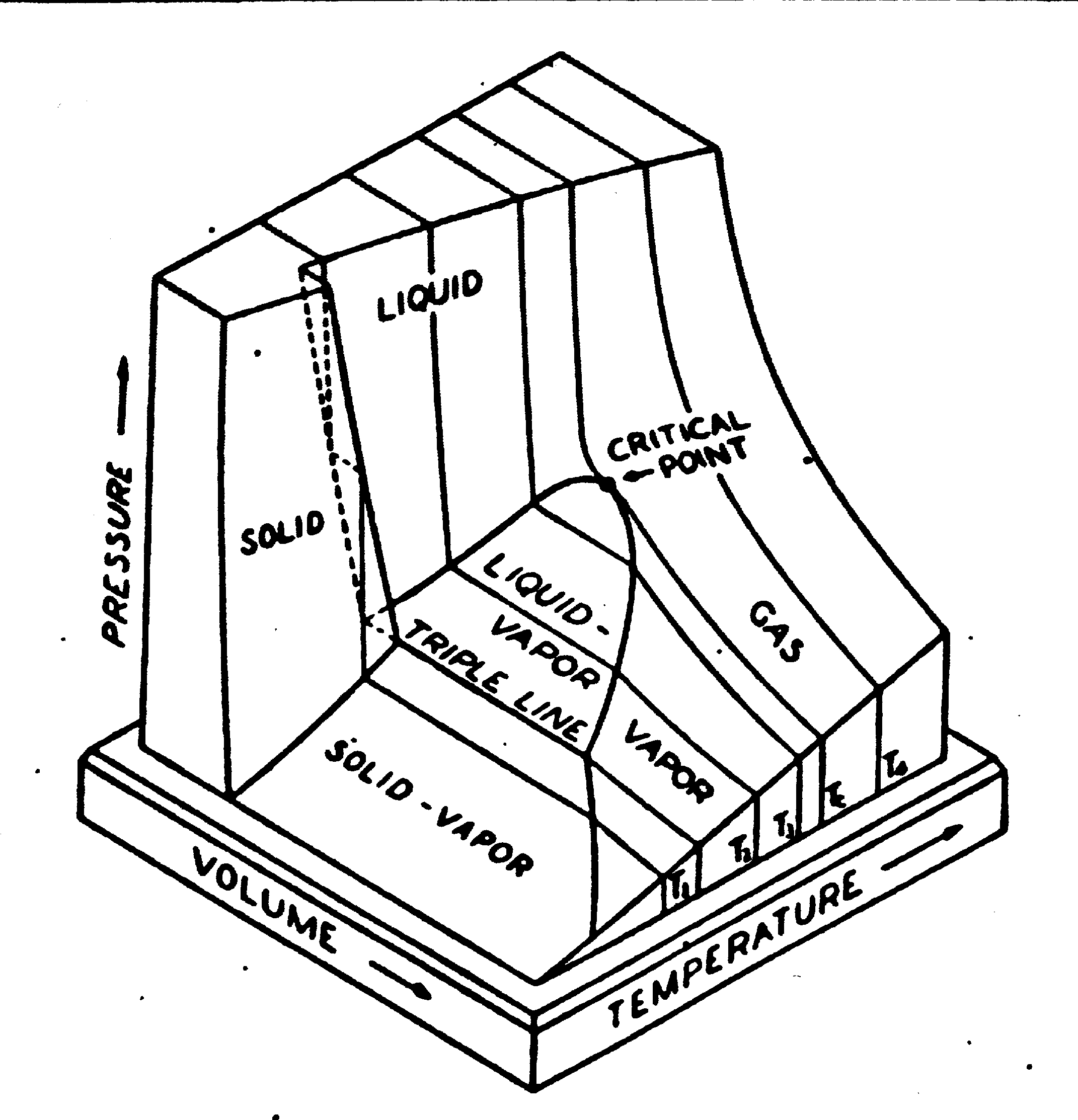
Otto cycle- regular gasoline
engine. This gas cycle is on the PVT surface... see if you can
sketch the cycle on the PVT surface (projection of the cycle is
shown below).
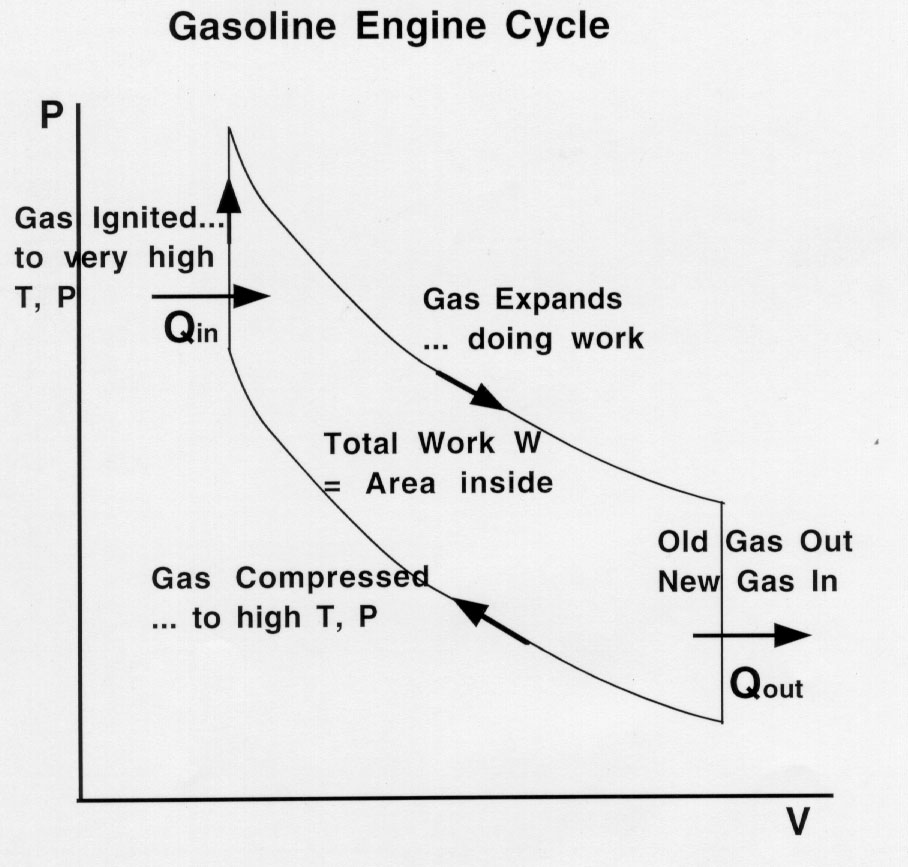
Gas and air mixture introduced
into piston at low pressure and high volume... piston then pushed
in rapidly (adiabatically = no heat transferred) by external force
on piston.
When gas fully compressed,
spark ignites gas to combust very rapidly (volume stays constant,
temperature and pressure increase).
Piston then pushed out rapidly
(adiabatically = no heat transferred) and does work for us.. this
is the "working stroke".
Piston then pushed in to get
rid of burnt gas and then pulls in new gas/air mixture to begin
new cycle.
Since the new gas is same each cycle, its internal
energy has no net change. Thus applying the first law of thermo:
Q - W = DKEInternal
This is true of all engines. Thus:
QIn - QOut - W =
0
QIn
- QOut =
W
Example: Suppose the chemical energy released
in the combustion released (QIn) 600 Joules of energy and produced a temperature (THot)
of 550 K. Suppose that 250 Joules of net work (W) is done in the
cycle. Then the heat that would be exhausted (QOut) would
be 350 Joules to the surroundings at a temperature (TCold)
of 300 K.
Recall that efficiency of engine is given by
Efficiency = Output (W) / Input (QIn)
= 250/600 = 42 %.
The maximum possible efficiency is determined
by the hottest and coldest temperatures given by
Max Eff = 1 - TCold / THot = 1 - 300/55 = 45 %
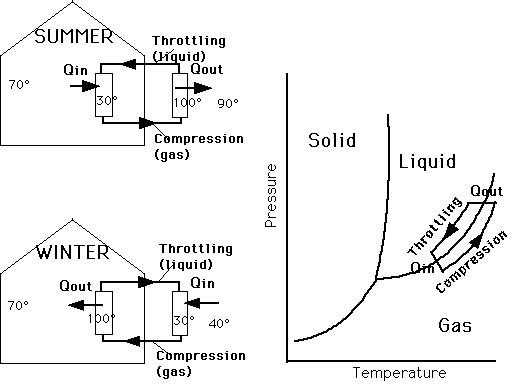
Application to Refrigerator,
Air Conditioner, Heat Pump.
We want heat to flow from a cold body to a
hot body
Refrigerator: from food to room
Air Conditioner: from cool room to hot outside
air
Heat Pump: from cold outside air to warm air
inside
Freon is the medium which is made colder (by
a throttling process) than the region where we want to take heat
out (food, cool room, cold outside air) and then hotter (by using
compressor) than the region where we want to exhaust heat (space
behind refrigerator, hot outside air, warm room).
The throttling process lowers the pressure
and temperature of the freon and causes it to go from liquid to
gas but heat is needed to be put into the freon as it crosses
the liquid-gas boundary.
The compressor increases the pressure and temperature
of the freon and causes it to go from gas to liquid but heat must
be taken out of the freon as it crosses the gas-liquid boundary.
This cycle is shown in the PT diagram and sample
of air conditioning a home in summer and heating a home in winter.