December 1, 1998
Recall the most basic Work / Energy Equations
WF = FPath Dx where FPath is amount of force F that is acting along path and Dx is change in position along path.
WS = DKE where WS is work done by total force along path and DKE is the change in Kinetic Energy (1/2 m V2final - 1/2 m V2initial)
WS has two terms in it that can be taken to the other side of the equation as change in energy.
Gravity Wgravity = -(mg hfinal - mg hinitial) = -DPEg
Spring Wspring = -(1/2 kx2final - 1/2 kx2initial) = -DPEs
Thus.... WS'' = DKE + DPEg + DPEs
Samples:

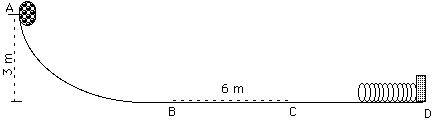
The object at the top of the curved, frictionless incline has a mass of 4 kg.
a) Relative to the bottom of the incline, how much energy does the mass have at point A.
b) How much kinetic energy will the object have at point B?
c) If the frictional force acting on the mass between points B and C is 2 N, What is the change in the kinetic energy of the mass between points B and C? Calculate work done by friction.
d) If the spring constant k = 800 N/m, how much will the spring be compressed when the object is brought to rest?
Now we want to include the energy of matter itself and the phases in which it exists.
The quantities we need to use are:
Pressure (P) = Force/Area P = F/A
Volume (V) = ADx
WF = FPath Dx = P ADx = P DV
Temperature (T) measures the average kinetic energy of the body.
<1/2 m v2> = 3/2 k T where k = 1.38 x 10-23 J/K
Since KE can be KEx, KEy, KEz then 1/2 k T
equals "packet of energy" for each "degree of freedom (f)" and we write the Internal Kinetic energy as U = f/2 k T
For monatomic atoms f=3, diatomic molecules f=5 or 6. For solids.. f=6.
There are two ways of changing the internal energy of a system: Work W done on system and Heat transfered into system.
Q + W = DU is the First Law of Thermodynamics
which can be added to WS'' = DKE + DPEg + DPEs to include thermal energy of a system to the total energy.
Relation between P, V, T
In gas phase: P V = n R T = N k T
PVT surface
PV view to see work done and heat added during change in PVT.
Engines
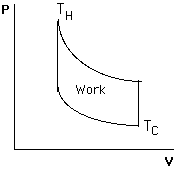
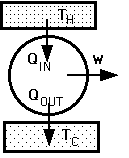
Takes in Heat QIN at hot temp TH, does Work W, exhausts Heat QOUT at cold temp TC
QIN = W + QOUT
Efficiency = Output / Input
= W / QIN = (QIN - QOUT) / QIN
Max Eff = (TH - TC) / TH
PT View
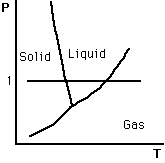
Isobaric heating of Water
Isothermal pressure increase
Refrigerator / Air Conditioner
Other Energy Notes
Chemical Bonding... Combustion
Biological Energy... Food
Solar Energy... Photons